Introduction: Oxygen is delivered to all tissues in the body through the hemoglobin molecule uniquely present in red blood cells (RBCs). Proper production and function of RBCs is therefore critical to human health. However, genetic defects of the hematologic system such as anemias and hemoglobin disorders (broadly termed “hemoglobinopathies”) are among the most common genetic disorders in the world. Therefore, developing strategies to regulate RBC development may allow researchers and clinicians to increase therapeutic potential of hematologic cell and gene therapies.
Erythropoietin (EPO) signaling is indispensable to RBC development. In its native form, two EPO receptor (EPOR) monomers dimerize in the presence of EPO cytokine to initiate a downstream signaling cascade. Prior work has shown that conformational requirements of EPOR dimerization are rather “loose” and signaling may also be initiated by agonistic diabodies or in the context of chimeric receptors. While these chimeric receptors have limited clinical applicability, inducible dimerization domains such as engineered FKBPs are currently being used in clinical trials in the form of inducible safety switches.
Due to the modular nature of cytokine receptors, we hypothesized that inducible dimerization domains (such as the FKBP domain) could be paired with endogenous receptors and signaling pathways to generate chimeric receptors that place RBC development under the control of a bioavailable small molecule - and therefore in the control researchers and clinicians.
This study presents a set of chimeric, synthetic receptors that support HSPC output towards the erythroid lineage via a non-immunogenic, non-toxic small molecule (AP30187, hereafter termed “BB”).
Methods: In-vitro editing was performed in primary human CD34+ HSPCs by electroporating Cas9 protein complexed with sgRNA along with an AAV6 DNA repair template to facilitate gene insertion via homology-directed repair (HDR). Bulk edited CD34+ HSPCs were then differentiated into RBCs using established differentiation protocols in the presence and absence of both erythropoietin and BB. Differentiation was determined by staining for RBC surface markers (CD71 and GPA) and subsequent flow cytometry analysis. Erythropoietic output was determined by cell density counts. Allele frequency of the edited cell population was determined through droplet digital PCR.
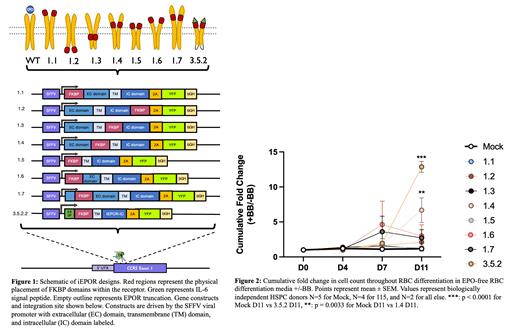
Results: We generated seven FKBP-EPOR chimeric receptors - with the basic structure of SFFV-[FKBP-EPOR]-2A-YFP-bGH - that placed the FKBP binding domain at different regions of the endogenous receptor (Figure 1) and analyzed their ability to support RBC differentiation in the absence of EPO but with BB. Out of the seven constructs we found a set of receptors - 1.4 and 1.5 - that demonstrated erythropoietin-independent, BB-mediated erythroid differentiation at 108% and 94% mock RBC differentiation respectively. Construct 1.5 - dubbed inducible EPOR (iEPOR) - was chosen over 1.4 for further optimization because it was also no longer EPO responsive.
We further optimized the design of iEPOR by 1) introducing a secretory signal peptide to improve trafficking of the receptor to the cell surface; 2) inclusion of a naturally occurring EPOR truncation that results in supraphysiological RBC differentiation; and 3) modification of the genomic insertion site and promoter of the iEPOR cassette to generate a toolbox of expression profiles.
Our results suggest that iEPOR mimics the native EPOR signaling cascade with high fidelity and sensitivity according to bulk RNA-Seq data. We also observe a significant increase in erythropoietic output for iEPOR version 1.4 (6.7-fold by day 11 of RBC differentiation in +BB over -BB conditions, P=0.003), which was further enhanced by the above modifications for vector iEPOR version 3.5.2 (12.9-fold by day 11 of RBC differentiation in +BB over -BB conditions, P<0.0001). Additionally, iEPOR is demonstrated to be erythropoietin-independent, dose-responsive, and tunable via expression from different loci and promoters.
Conclusion:iEPORs represent a powerful new tool to alter the cellular dynamics of red blood cell development. The work demonstrated in this study has the potential to dramatically improve the efficacies of existing gene therapies, offer a route to EPO-free ex-vivo RBC production, as well as serve as a new tool by which to better understand stem cell biology and differentiation.
Disclosures
Porteus:Kamau Therapeutics: Current equity holder in private company; Alaunos Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Allogene Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Graphite Biologics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; CRISPR Tx: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal